Advanced support with
high added value
Building Information Modelling
4CE team specialists are able to manage the integration of the medical design component in the BIM modelling. The furniture, fixture and equipment lists (FF+E) for each room are linked to their graphical representation, including, for the different families, the main information related to each item and their standard pre-installation requirements. Thanks to this approach, it is possible to check the consistency and the clashes between the medical and the other structural, architectural and MEP models. In particular, 4CE team is partner for Italy and official user of CodeBook®, a dedicated hospital design software that is compatible with the most common BIM applications.
Clinical engineering and facility management
Throughout the years of experience in health technologies management inside hospital infrastructures, both in Italian and international projects, 4CE team has developed its own roots from the clinical engineering sector. Its specialists are able to perform all the typical activities of this field: from maintenance plans to preventive and corrective activities management, up to producing complete renewal plans for the equipment. Furthermore, thanks to the activities directly conducted in hospital construction projects, 4CE engineers can efficiently support the optimization of the health facility management set up thanks to a long term perspective. This approach is implemented since the preliminary design phases by considering the medical technologies implications in the life cycle of the new facility, and it is carried on until the maintenance activities set up and start up.
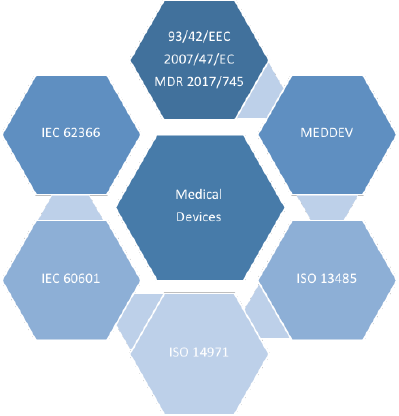
CE Marking of medical devices
4CE team is able to support companies involved in the manufacturing, commercialization and service of medical devices, to define the required activities and technical documentation needed to obtain the CE Marking, according to the European Union directives and the new Medical Devices regulation. 4CE engineers can follow the full certification process, starting from the devices classification up to the final delivery of the complete technical dossier, including the management of specific activities such as the risk analysis, clinical evaluation and post-market monitoring. 4CE team can also support companies along the certification process of their quality system and its related procedures, following the ISO 13485 medical sector standard..
High complexity Departments
For some units and services, medical equipment planning requires a thorough knowledge of the linked workflows and the related architectural, structural and installations implications. 4CE engineers have developed these competences¬¬¬¬¬ by successfully completing the following complex departments in different hospital projects: clinical laboratories, blood transfusion centers, central sterilization services, radiotherapy, nuclear medicine, operating theaters with hybrid and integrated rooms.
Medical Gases
Thanks to a field experience in many hospital construction sites and thanks to a professional certification as authorized expert according to the ISO 7396 standard, 4CE team is able to support the client in the strategic choices concerning the distribution of medical gases inside the different departments and buildings of the health facility. Furthermore, with an operational understanding of the clinical use of gas installations, 4CE engineers can interact with the manufacturers and the distributors in order to optimize the medical gases system according to the expected real workflows and the related equipment utilization. This support can then be extended to the choices on the type, capacity and location of the sources at the central gas station and to the possible on-site production systems of medical oxygen.
Commissioning Process
4CE team is engaged in the dissemination of the “commissioning process” methodology applied to healthcare technologies, inside hospital construction projects. ASHRAE 202-2013 (for buildings and systems) standard, conventionally adopted in the HVAC sector, is also suitable to conduct the medical engineering activities from the planning phase t and equipment installation, until testing and maintenance start up, in coordination with the commissioning authority in charge. Starting from the project clinical needs and operational requirements, translated into OPR (Owner Project Requirements) and BOD (Basis of Design), the commissioning process (CxP) facilitates an integrated approach of the medical equipment with the other infrastructural and installations components, thanks to a total building commissioning approach, in order to guarantee the correspondence between results and expected technical and functional goals for the new healthcare facility.
Leed
The environmental certification according to LEED protocol (Leadership in Energy and Environmental Design) is getting more and more utilized in Europe and in Italy, becoming the referral standard also in hospital construction projects. The medical component, though, is not typically considered in this kind of processes. 4CE team is at the forefront in this field, believing that medical equipment can significantly contribute to the environmental sustainability and the energy efficiency goals of the new health facility. This could be achieved, for the BD+C (building design and construction) rating system of LEED v4 in its healthcare declination, through an appropriate identification of the credits and prerequisites categories, that are also suitable to healthcare technologies, intended as an integrated system to the building and its installations.

Green Public Procurement
With the recent introduction of the new public tendering code and the related European indications on green procurement for public administrations, the environmental aspects management during the tender and the evaluation process of biomedical technologies, becomes even more strategic and critical. 4CE team is already working in this area through specific research projects which have allowed to investigate the opportunities for medical equipment procurement, at national but also at European level, in terms of GPP (Green Public Procurement) tools and CAM (minimum environmental criteria). 4CE engineers are able to support the contracting authorities in the identification of the appropriate green criteria and requirements, in order to ensure a procurement process aligned with the current Directives.
PPP e MES technology partnership
Among the most relevant experiences of 4CE team, there are several Project Financing participations in healthcare facilities projects, in which 4CE engineers have significantly contributed to the achievement of a successful public-private partnership. In this specific sector too, a 360 degree perspective combined with an interdisciplinary approach, applied since the initial needs assessment stage and until the medical equipment commissioning and management phase, are fundamental elements for the future efficient operability of the new health facility and its biomedicl technologies. Similarly, in MES projects (Managed Equipment Services), intended as PPP form specifically focused on high medical technologies, 4CE team can support the definition of the technical requirements and the related services, for a correct implementation of the technological partnership.
Projects verification
Thanks to the experience developed in medical design activities, 4CE engineers are able to support accredited third party assessment firms in projects verification and public administrations in the validation stage. 4CE team is able to check the compliance of the design with respect to specific medical technical and functional prescriptions, in an integrated modality with the other disciplines (structural, architectural and MEP), for all the formal design levels required by law. This verification is advantageous for contracting authorities even when not mandatory, as it is meant to guarantee the matching between the medical planning and the other disciplines design, hence mitigating the risk of increase of budget costs due to claims or delays in works execution.
International Cooperation
Several 4CE team members have worked for many years in the humanitarian and development sector, in healthcare projects and construction of hospital facilities, in developing Countries and emerging economies. 4CE engineers can support NGOs, international organizations and governmental institutions during critical stages such as reconstruction in the aftermaths of conflicts or natural disasters, protracted emergencies, recovery and early development. The deep knowledge of the technical and logistics aspects of healthcare projects on one side, and the understanding of the context and its organizational implications on the other, are the fundamental elements for the success of projects, where it is strategic to adapt the engineering approach to the local needs, while maintaining at the same time a high qualitative level.